Hydrobromic Acid 48%
Home » Products » Liquid Bromine and Derivatives » Hydrobromic Acid 48
Hydrobromic Acid 48%
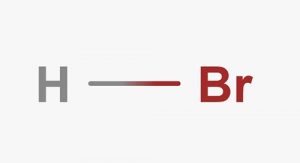
Symbol – HBr
Molecular Weight – 80.91
CAS No – 10035-10-6
Hydrobromic acid is a strong acid formed by dissolving the diatomic molecule hydrogen bromide (HBr) in water. “Constant boiling” hydrobromic acid is an aqueous solution that distills at 124.3 °C and contains 47.6% HBr by mass, which is 8.77 mol/L. Hydrobromic acid has a pKa of −9, making it a stronger acid than hydrochloric acid, but not as strong as hydroiodic acid. Hydrobromic acid is one of the strongest mineral acids known.
Physical Properties
Appearance | Liquid |
Melting point | -11⁰C |
Boiling point | 126⁰C |
Density | 1.49g/cu.cm |
Solubility | 204 g/100ml water @15⁰C |
Uses
- Hydrobromic acid is mainly used for the production of inorganic bromides, especially the bromides of zinc, calcium, and sodium.
- It is a useful reagent for generating organobromine compounds.
- Industrially significant organic compounds prepared from hydrobromic acid include allyl bromide, tetrabromobis(phenol), and bromoacetic acid.
- HBr almost uniquely participates in anti-Markovnikov hydrohalogenation of alkenes. The resulting 1-bromoalkanes are versatile alkylating agents, giving rise to fatty amines and quaternary ammonium salts.
Handling & Safety
- Keep container tightly closed and protected from physical damage.
- Use chlorinated vinyl chloride resins or polyethylene glass coated equipment.
- Keep container in a cool, well-ventilated area away from incompatible materials.
Packaging
- 340 Kgs HDPE Drums.
- 1700 Kgs IBC Tanks.
Molecular Structure